Hello everyone,
Here is a link to my powerpoint presentation.
Russell PowerPoint
Russell
Friday, April 22, 2016
Sunday, April 17, 2016
ABS and Charts
Hi everyone,
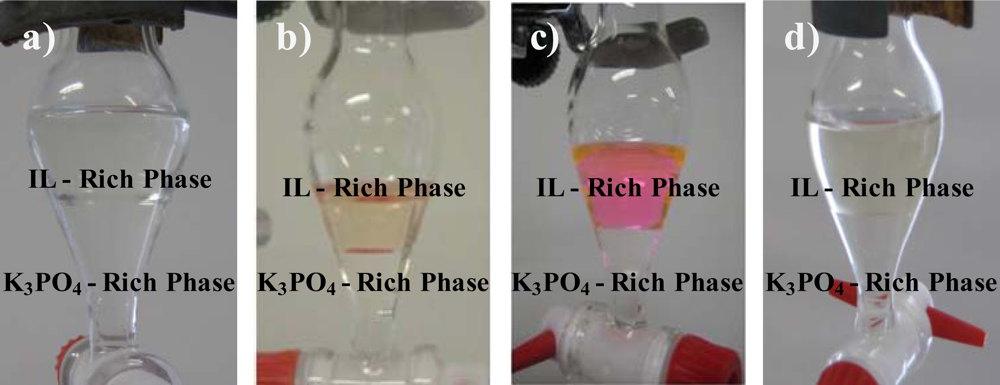
This past week I began mixing solutions to see if I could form aqueous biphasic system (ABS). An ABS is simply when a solution is mixed and after a while, two aqueous layers are formed. This is relevant to my project as the one compound that significantly affects release forms an ABS when mixed with the polymer. Here is a very terrible picture of that ABS.
It can be seen as ABS because there are two aqueous phases, the yellow part at the bottom and the clear part on top of it. Here are better pictures of ABS's that I found online.
(http://www.mdpi.com/1422-0067/11/4/1777)
To simply practice how I plan on doing this and double check my past results, I formed mixtures of all the salts I have used previously and the polymer that forms the hydrogel. As expected, none of the previous materials I used ABS's.
After this, I began doing some reading on what compounds form ABS's and will try various different compounds over the next few weeks.
On Friday last week, I also finally processed the data from two more release studies I performed! The first graph shows a release study where I greatly increased the concentration of acid from the last release study I posted a graph about, and the second graph shows a release study where I doubled the concentrations of acids and salts from that previous release study.
Both of these graphs are very similar to the previous release study I performed: there is some difference between the acids and salts and the control. However, that difference is not nearly as large as the difference with the gel mixed with a drug. And that release profile of the gel mixed with drug is really the goal for this study.
Thanks for reading!
Russell
Saturday, April 9, 2016
A Different Approach
Hi everyone,
This week, I started another release study with double the concentration as the previous release study. Because I was not feeling well on Friday, I was unable to process the data, but that information will be included in my post next week.
I also prepared for another release study with high concentrations of acids. I couldn't use salts because larger amounts of salts would not dissolve into solution. Hopefully, I can have this data next week as well.
These last two release studies hopefully support my previous data or show a correlation between the acid or salt concentration and release times.
As I finish these two release studies, I will begin a different approach to answering my research question of ways to modify drug release.
The new approach is based off of an observation: certain salts or drugs cause biphasic systems to form when mixed with the gels. A biphasic system is simply when a solution has two distinct parts. The hypothesis is that the more likely a compound is to form a biphasic solution, the faster the release will be.
To test this, I will simply mix many compounds together with a particular gel and see if a biphasic system is formed.
See you next week,
Russell
Monday, April 4, 2016
More Data
Hi everyone,
This past week, I accumulated much more data, primarily from the release study I started the week before.
For this study, I mixed the polymers with drugs and a salt or acid. The acids I used were sulfuric acid and hydrochloric acid. The salts I used were sodium chloride and sodium thiocyanate.
Here is a graph showing the amount of drug released at the following time points: 0 hour, 1 hour, 6 hours, 24 hours, and 48 hours. The "control" is the gel without acid nor salt.
compared the sizes of the gels to their release profiles. I did this because those gels this time did not look very good as the gel broke apart in the vial (probably because I did not get rid of all the bubbles in the syringe). Although their components and masses are the same, some conditions may have been different, and so there can be some doubt on those results.
Nevertheless, the results do show a particular trend with the additions of different salts or acids, and that's a really good step forward in this research project. Next week, I plan to start another release study with the same materials but at double the concentration. Hopefully those results are similar and support the results from this release study.
Till next time,
Russell
This past week, I accumulated much more data, primarily from the release study I started the week before.
For this study, I mixed the polymers with drugs and a salt or acid. The acids I used were sulfuric acid and hydrochloric acid. The salts I used were sodium chloride and sodium thiocyanate.
Here is a graph showing the amount of drug released at the following time points: 0 hour, 1 hour, 6 hours, 24 hours, and 48 hours. The "control" is the gel without acid nor salt.
Firstly, positives: the shape of this curve is very similar to the curves of release studies in the past. Furthermore, almost all of the curves have the same shape. Also, in the first release study, my data suggested that the gels were not mixed well. However, this time, that was not the case as the gels from the same syringe had similar release data, as shown by this small part of my document.
Now, some not so great things. The last two series (the control and the .128mmol salt) are actually not from this release study. Rather, they are from the release study I did before this, the one where Icompared the sizes of the gels to their release profiles. I did this because those gels this time did not look very good as the gel broke apart in the vial (probably because I did not get rid of all the bubbles in the syringe). Although their components and masses are the same, some conditions may have been different, and so there can be some doubt on those results.
Nevertheless, the results do show a particular trend with the additions of different salts or acids, and that's a really good step forward in this research project. Next week, I plan to start another release study with the same materials but at double the concentration. Hopefully those results are similar and support the results from this release study.
Till next time,
Russell
Subscribe to:
Comments (Atom)




